FDA Value Proposition for Software Development
Project Overview:
Data Base Verification (4-Week Agile Sprint w/Daily Stand-Up)
Client:
Federal Drug Administration
Design Technology Used
Adobe Illustrator, InDesign, Keynote, Sketch, Design Thinking Session, & Atlassian Jira Agile Project Management
Release Date:
Pending project definition status
Project Overview:
The initial stages of this design sprint began with an investigation into email message communication between prominent keepers of information. The importance of minimizing wasted efforts in data verification was a critical time-on-task tracking point.
Problem/Challenge:
The situation inside the FDA was unique in several ways, primarily that software design and development engineering did not understand their user community. The challenge was how to bring end-user issues to the forefront user-centered-design techniques.
Solution/Results:
Insight validation was conducted through a one-day design thinking workshop. The design thinking workshop brought together individuals with varied backgrounds and viewpoints enabling breakthrough insights and solutions to emerge from the initial design scope. This process was the essential tool that illuminated the desire for using systematic reasoning and intuition to explore ideal state software development within the FDA. The collaborative effort helped to formalize and disseminate the UX communication practice with success metrics that supported an emergent design language. Those success metrics were established with these definitions: personas, use case definitions, experience mapping, scenarios, end-to-end user journey map, functional flows, sketching those moments with conceptual wireframes, and prototype demo. We encapsulated the core moment needs of the analyst persona while delighting the client with moments of truths.
Typography & Visual Design:
The aspects typography and product visual design were not addressed at this stage of wireframe implementation.
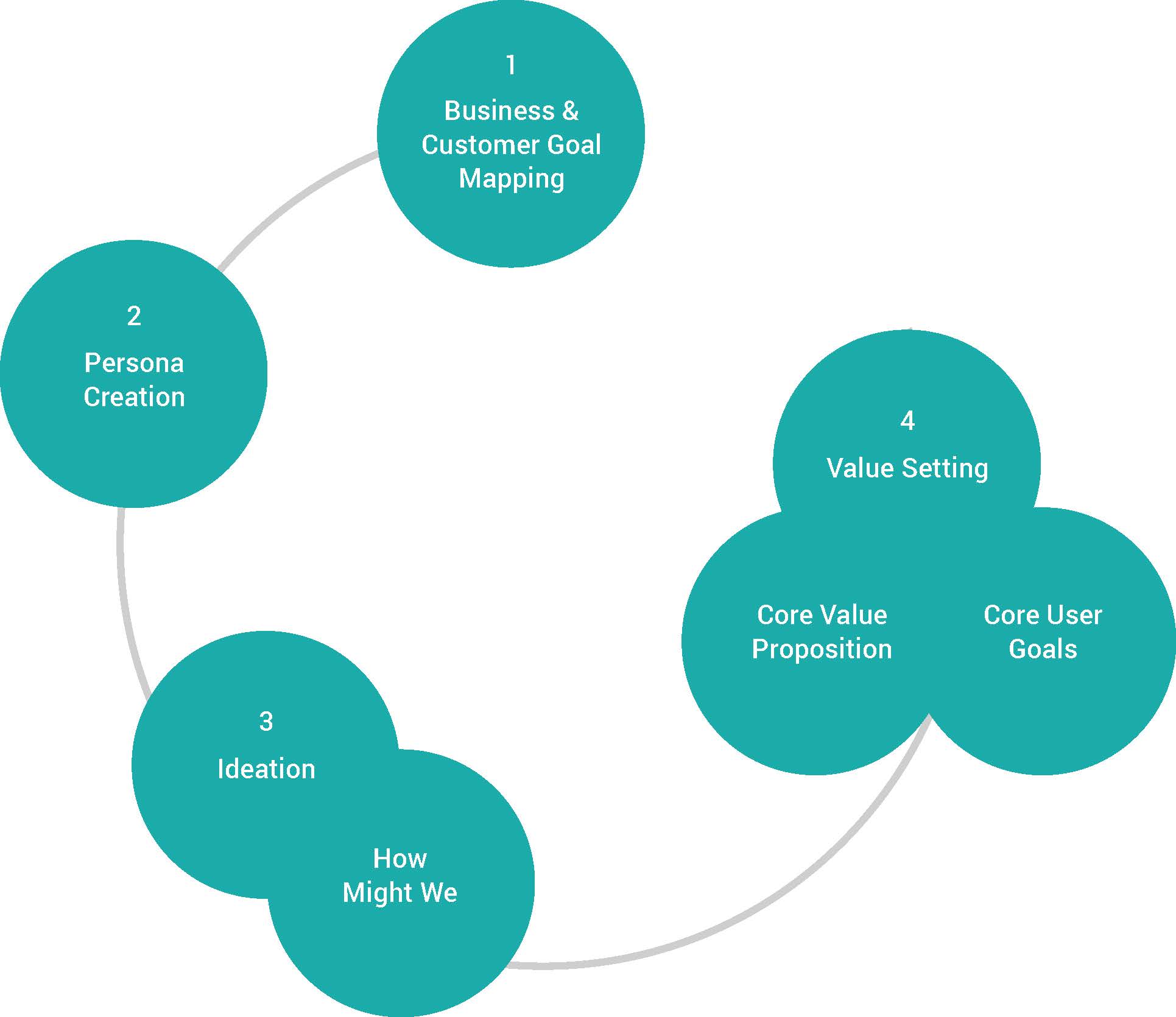
Goal Mapping:
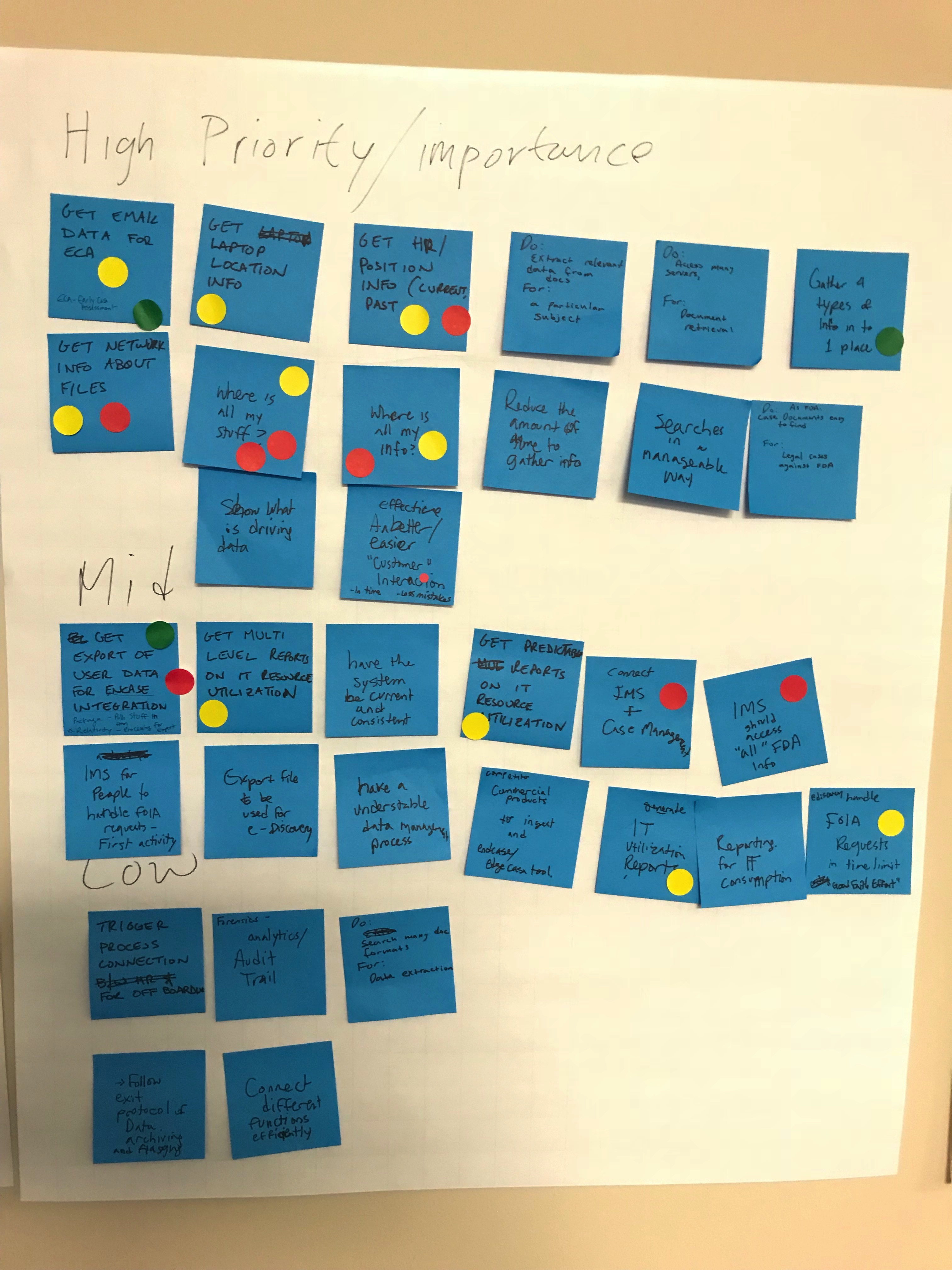
This process involved stakeholders from both business management and end-users using post-its and colored dots as selection methods. The interaction between the participants provided insight into how collaboration within the solution space offered ideas yet to be explored.
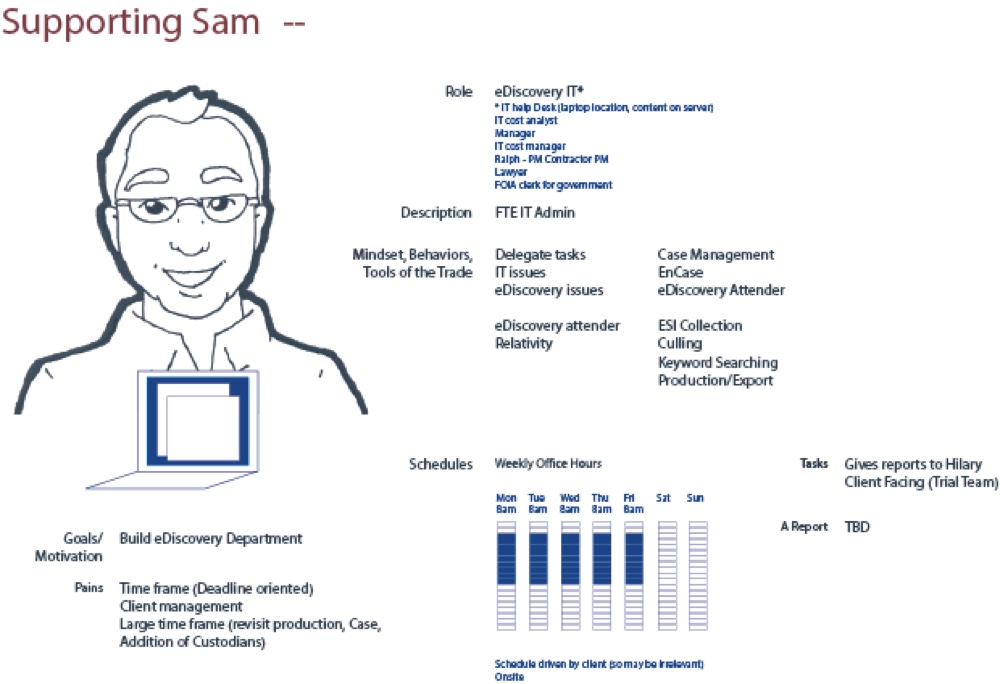
Persona Development:
During our workshop, personas were created based on input from the participating individuals. Four personas were created - Supporting Sam and Analyzing Alice were identified as the pivotal individuals in the business flow.
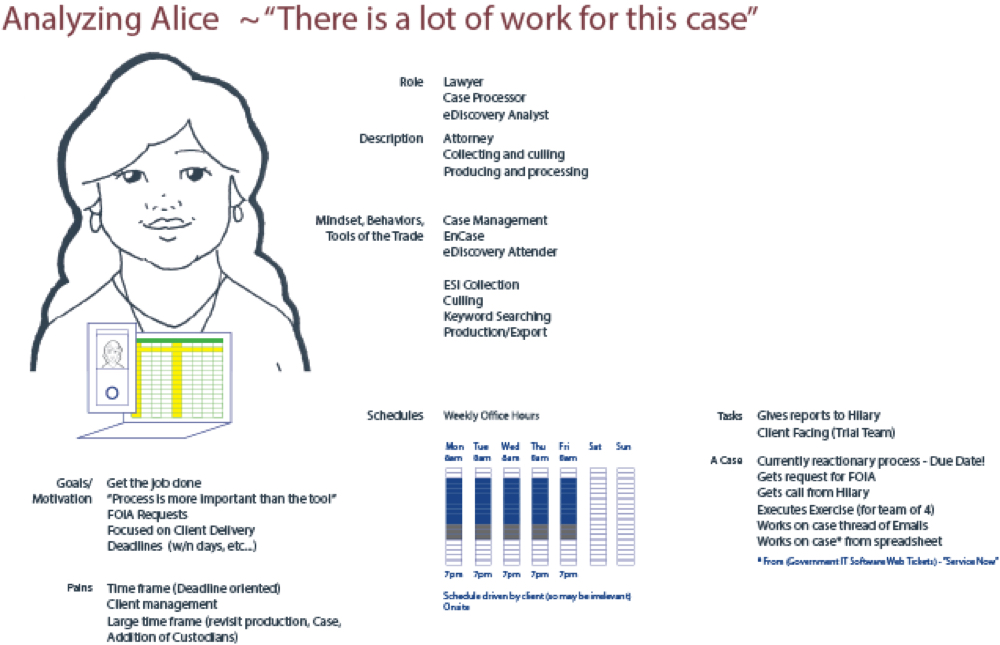
Value Setting - Core Propostion - Core User Goals
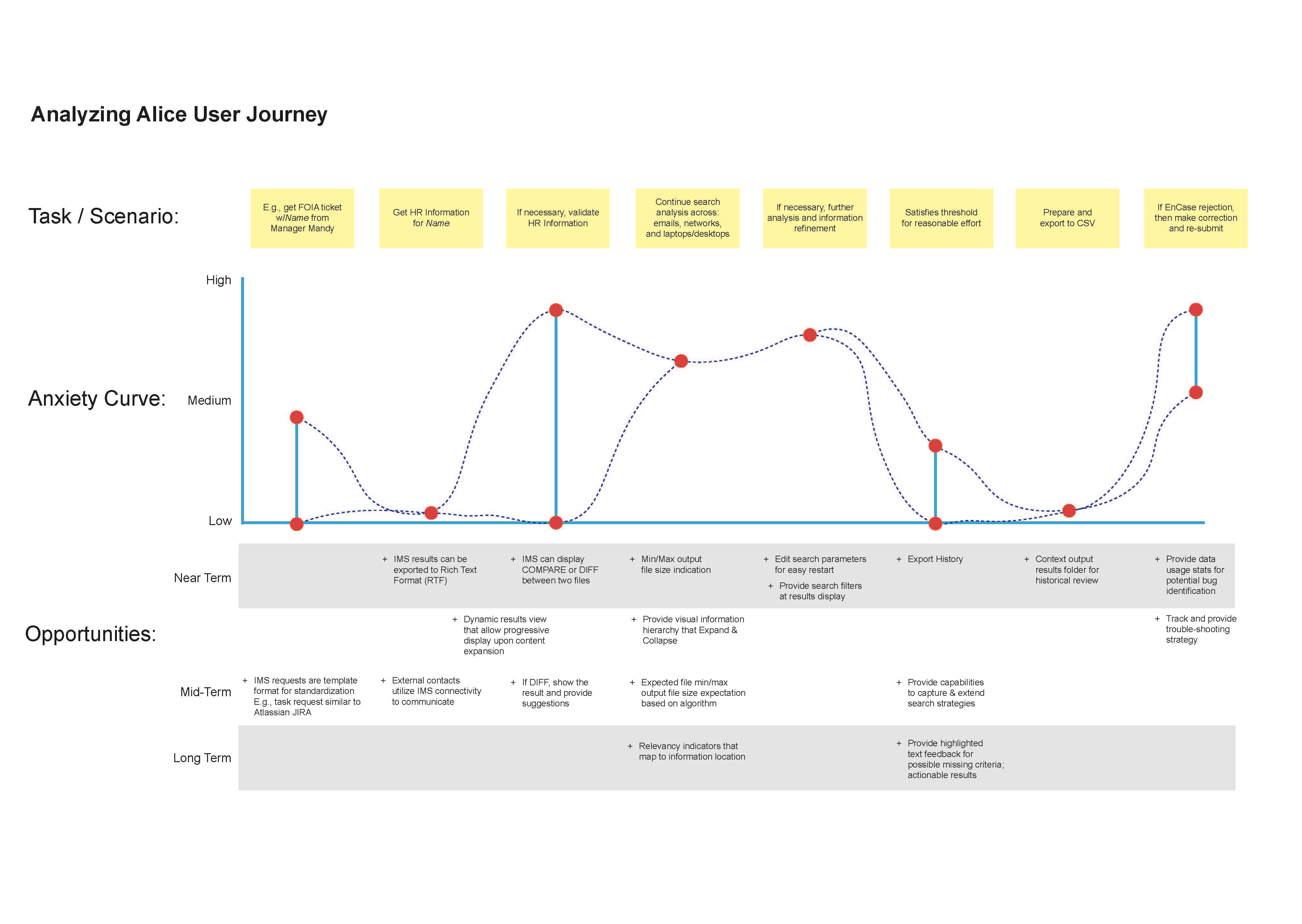
These primary points of interest are captured in the user journey map as shown below. As indicated, common themes emerged in the anxiety curve where stress level at key data points pushed individual performance boundaries. Where completing tasks in a timely manner, while collaborating with others to maintain deadline target dates are key motivators for the analyst. The importance of reasonable effort to gather all points of data connections is highligthed in the goals/expectations journey map below.
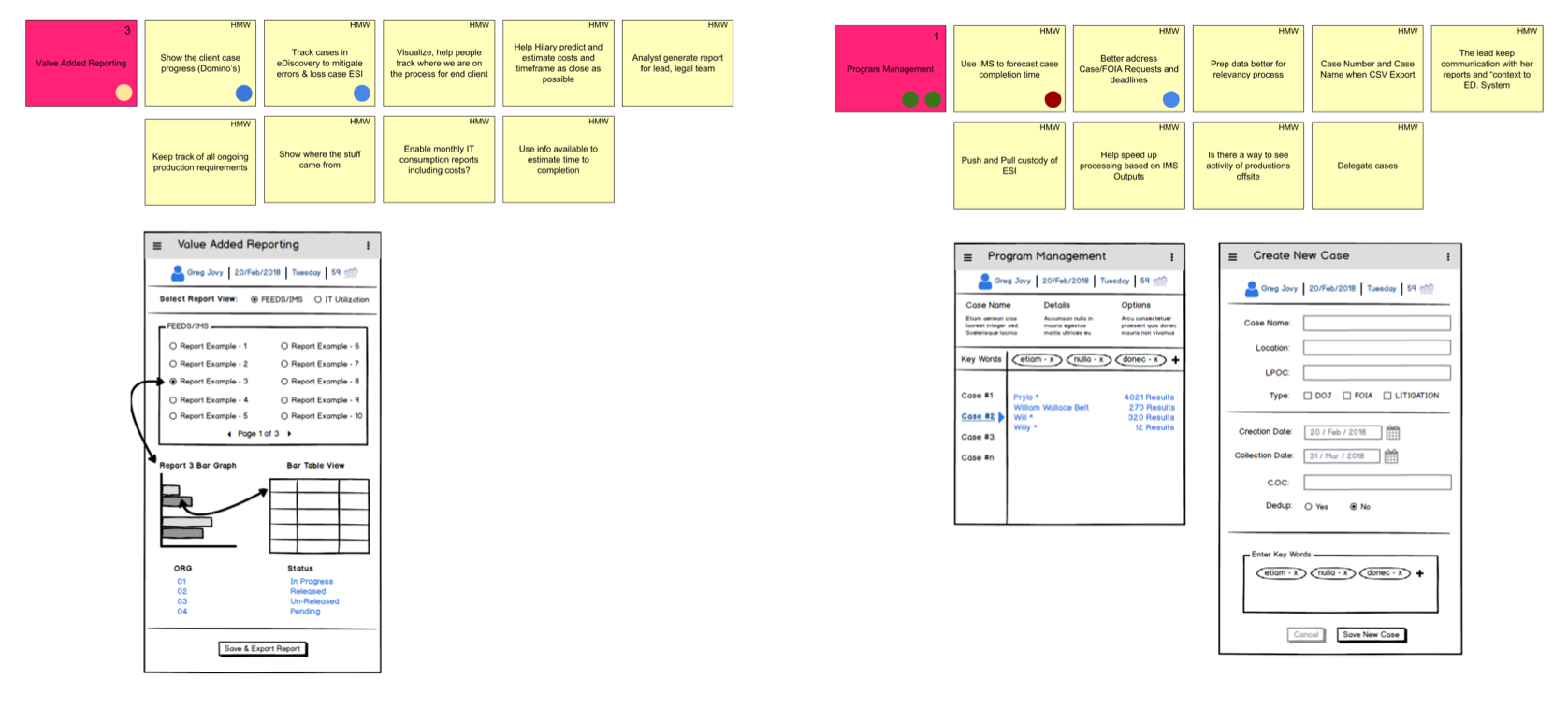
Ideation How Might We
The ideation lens of “how might we” opened the door to the possibilities using real-time data discovery due diligence descriptors. Participants provided a primary focus on their values of how the system should solve their problems. In doing this, the establishment of a fundamental baseline for design evolution took shape. We explored the hows and the whys of the problem and outlined their expectations.
The below views represent recorded ideation data points and goals where the participants directed the context of information organization.
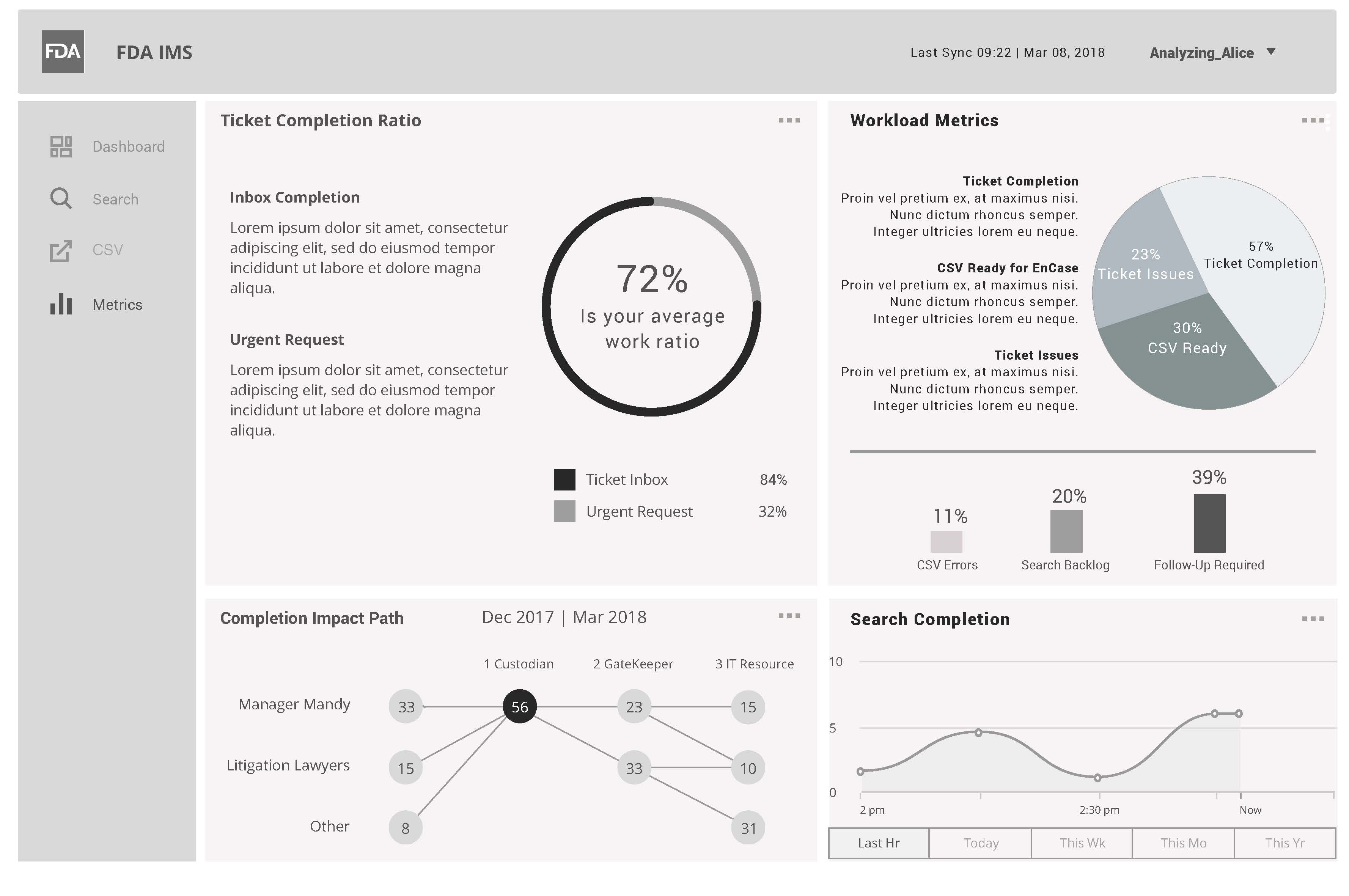
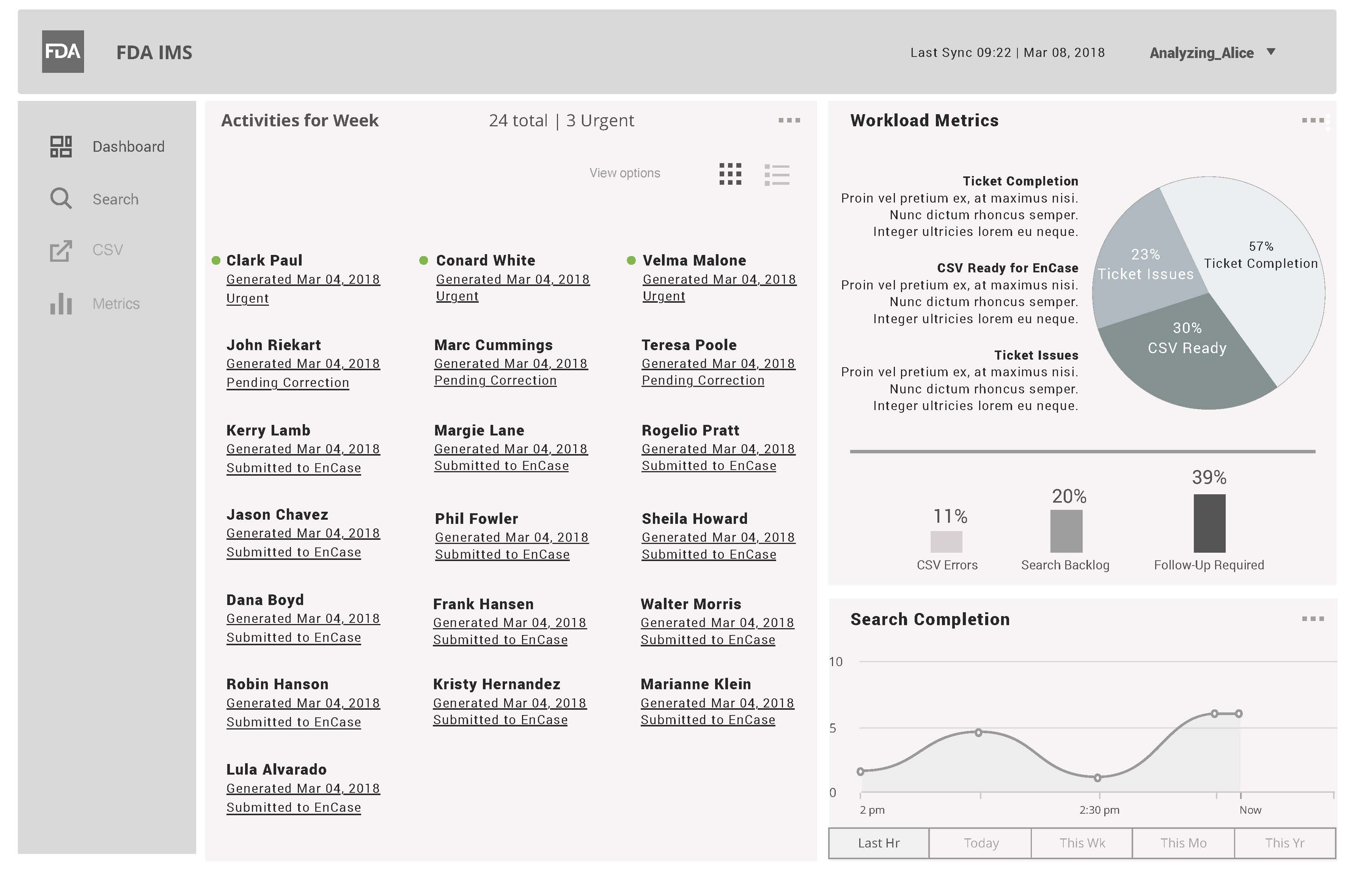
FDA IMS Dashboard View
This wireframe contains metrics based on Analyzing Alice actual throughput per name search.
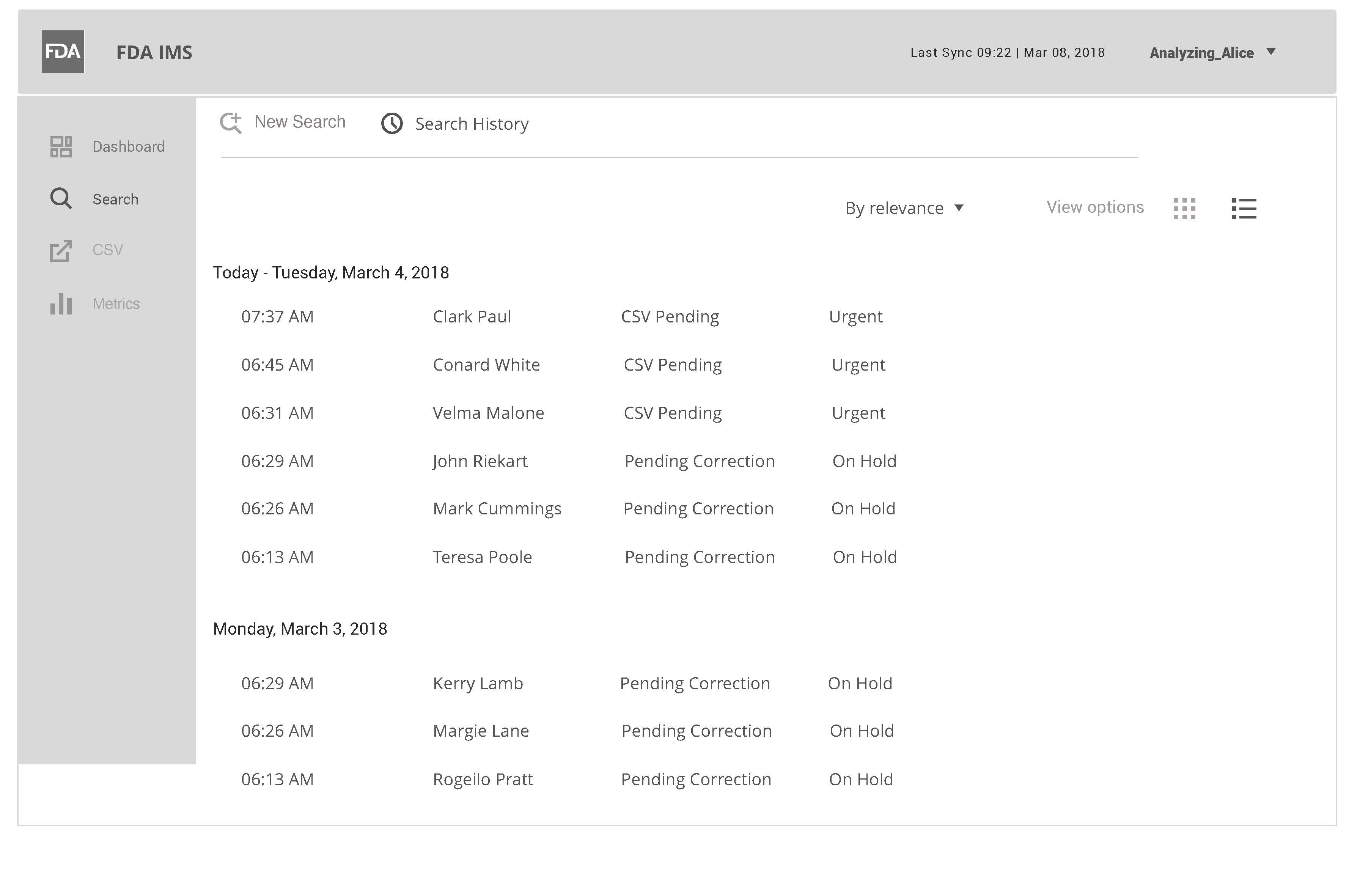
IMS Search Results View
This wireframe represents a daily search with filter capability.
IMS Metrics View
This wireframe contains cumulative diagrams based on work completed by the analyst.